The Fluxergy Test Kit COVID-19, which utilizes state-of-the-art Sample-to-Answer-RT-PCR and microfluidics technology, has been shown to identify the SARS-CoV-2 virus in one hour. This device is solely intended to be used by healthcare professionals. Results were obtained from internal performance evaluation using clinical samples benchmarked against an FDA-EUA approved method.
FAST PCR: DETECT SARS-COV-2
IN ONE HOUR

HOW TO RUN A FLUXERGY PCR
Fluxergy’s COVID-19 test, which utilizes state-of-the-art PCR and microfluidics technology, has been shown to identify the SARS-CoV-2 virus in one hour. See how easy it is to run a PCR test in your lab with Fluxergy’s compact analyzer.

Place Prepared Sample into Fluxergy Card

Insert Fluxergy Card into Fluxergy Analyzer

Get PCR Results in One Hour
SIMPLE SAMPLE PREPARATION
WITH NO EXTRACTION REQUIRED
Viral Transport Media

Vortexer (Not Included)

Fluxergy Reaction Mix

Mini Centifuge

Fluxergy Card

Fluxergy Analyzer
Fluxergy Works (Computer not included)
RNA extraction is NOT required. The Fluxergy Test Kit COVID-19, which utilizes state-of-the-art Sample-to-Answer-RT-PCR and microfluidics technology for a simplified workflow.*
*Refer to Instructions for Use for complete user instructions for the Fluxergy Test Kit COVID-19. This device is solely intended to be used by healthcare professionals. Rev. 3-24-21
DETAILED SPECIFICATIONS
FLUXERGY TEST KIT COVID-19
| Assay Specifications | |
|---|---|
| Test Type | RT-PCR, Direct |
| Time to Result | ~55 minutes |
| Sample Preparation | ~3 min from reagent thaw, No extraction required |
| Sample Type | NPS in 3mL VTM, See accepted VTMs* |
| Required Sample Volume | 14µL |
| Storage Condition | Fluxergy Card COVID-19: 10° to 30°C Fluxergy Reaction Mix COVID-19: -10° to -30°C |
| Gene Targets | Orf1ab polyprotein and N gene; Detects B.1.351 and B.1.1.7. variants (does not differentiate) |
| LOD* | 0.89 TCID50/mL |
| Platform Specifications | |
|---|---|
| Analyzer Size | 26.19 cm x 13.13 cm x 25.80 cm |
| Analyzer Weight | 6.8 kg |
| Power Input | 12V DC, 7A |
| Operating Temperature | 15°C – 30°C |
| Storage Temperature | 15°C – 30°C |
| Operating Humidity | 10%-85% |
| Minimum Computer Requirements | Operating System: OS must be 64-bit, Windows 10 v1151 Processor: Intel Core i5 2.5GHz or equivalent Ram: 8GB DDR4 HDD: 250GB Screen: 1080p USB: 2x2.0 port (for scanner and mouse) Networking: Ethernet port |
| Data Analysis Tools | Qualitative Result Interpretation, Manual Amplification Curve Review |
*Refer to the Fluxergy Analyzer Instruction Manual for full specifications. This device is solely intended to be used by healthcare professionals. Rev. 3-24-21
TEST RESULTS
In a real-time PCR assay, a positive detection of target nucleic acid is confirmed by accumulation of a fluorescent signal after the specific target DNA is amplified. Fluxergy Works software will output a qualitative positive or negative result from your PCR test. This is determined by sensors that look for amplification/presence of the target gene (solid line) and an internal control target (dashed line). The internal control target monitors sample integrity and system functionality.

Positive Result
Both the target gene and positive control are amplified.
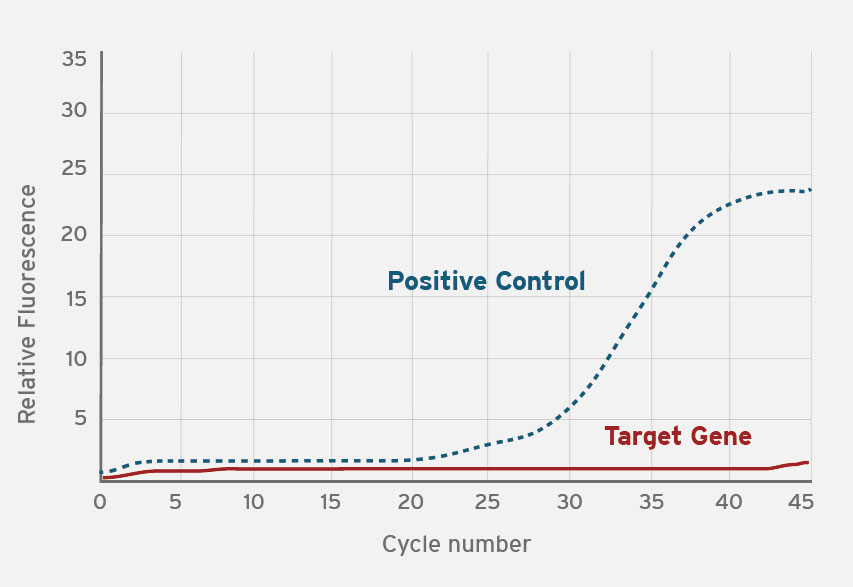
Negative Result
Only the positive control target was amplified.
COMMITTED TO COMPLIANCE:
CE-IVD, ISO 13485:2016, AND MDSAP
Fluxergy has obtained CE marking for its one-hour COVID-19RT-PCR test, to use by healthcare professionals as an in vitro diagnostics (IVD) for the detection of SARS-CoV-2. The CE-mark will allow Fluxergy’s innovative testing platform to enter the European Union market and any other markets that accept CE-marking as valid regulatory approval.
In December 2020, Fluxergy received dual ISO-1 3485:201 6 and MDSAP certifications. ISO 1 3485:201 6 is the medical device industry’s most widely used international standard for quality management systems to design, develop, produce, and deliver products. MDSAP certification satisfies the quality system requirements of the regulatory authorities participating in the program, including the U.S. Food and Drug Administration (FDA), Health Canada, Brazil’s Agência Nacional de Vigilância Sanitária and Australia’s Therapeutic Goods Administration.


“Fluxergy is dedicated to producing tools that make health information accessible and available to everyone. We are focused on bringing our unique diagnostic products to market rapidly and innovatively, ensuring our end-users always have a product with the latest technology while maintaining the highest level of quality.
We do this through a data-driven process that focuses on continuous improvement.
Fluxergy is seeking commercial partnerships with healthcare providers and potential international go-to-market partners in Europe, Asia, and Australia”
– Dr. Ali Tinazli, Chief Commerical Officer
 de_DE
de_DE en_GB
en_GB